Guy’s and St Thomas’ treats first patient in UK with life-changing gene therapy treatment
Thursday 19 June 2025

The team involved in the UK first
Guy’s and St Thomas’ has become the first Trust in the UK to treat a patient on the NHS with a new, potentially life-changing drug for haemophilia B.
Haemophilia B is an inherited bleeding disorder caused by a lack of Factor IX, an important blood-clotting protein. Patients often experience spontaneous bleeding leading to chronic joint pain. Up to now, these patients face the burden of regular infusions of clotting factors one to twice a week.
Hemgenix (etranacogene dezaparvovec), a one-off gene therapy infusion lasting 1-2 hours could eliminate the need for regular injections of factor IX and prevent patients from having to experience painful bleeds.
The therapy works by introducing a working version of the factor IX gene into the body, enabling the liver to start making its own factor IX, and therefore reducing bleeding episodes.
The first patient to receive Hemgenix is from the North East of England and was diagnosed with haemophilia B whilst still a young child.
They said: “I've always had to be more cautious and to plan ahead. There is a level of anxiety in that and being overly cautious has often led to missed opportunities and things I can’t do, like contact sports. Being free from the burden of knowing I have the condition will be fantastic.
“To experience life free of the worry and to do things that I wouldn't normally do will also be amazing. Not needing to plan ahead for treatment deliveries or looking up hospitals in foreign destinations when going on holiday, or having to tell people ‘sorry I can't do that I've got haemophilia’, will be something I've always dreamed of.
“I really appreciate everyone involved in my care, from the team in London right now, and in Newcastle who have helped and supported me over the years with my condition. I couldn't have asked for more than all they have done.”
Dr Pu-Lin Luo, consultant haematologist at Guy’s and St Thomas’, said: “This is a big step forward in our ability to manage haemophilia B and could change the lives of some of our patients. It is also a testament to the advancement of cell and gene therapies in the UK and these are exciting times.
“Administering the first dose of Hemgenix outside of a clinical study was made possible by exceptional cross-centre collaboration between Guy’s and St Thomas’, where the patient was treated, and Newcastle Hospitals, their local haemophilia centre.”
Hemgenix will be available on the NHS as a treatment option for adults with moderately severe or severe haemophilia B. It has been funded by NHS England via the Innovative Medicines Fund (IMF).
Approximately 2,000 people in the UK have haemophilia B and the National Institute for Health and Care Excellence (NICE) estimates that around 250 will be eligible for the new treatment in England.
This medicine is subject to additional monitoring. This will allow quick identification of new safety information.
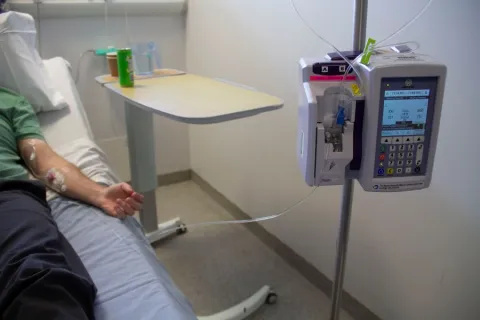
Patient receiving the gene therapy infusion
Last updated: June 2025
Contact us
If you're a journalist and have a media enquiry, please contact us.
Phone: 020 7188 5577
Email: [email protected]



