UK first as schoolboy has dissolvable fix for hole in his heart
Tuesday 3 February 2026

James Hoddinott
An 11-year-old boy has become the first person in the UK to have a hole in his heart repaired using a biodegradable device which gradually dissolves inside the body. The innovative operation took place at Guy’s and St Thomas’.
James Hoddinott, who dreams of becoming a marine biologist, is determined to learn to scuba dive in open water. But he was born with a hole in his heart, which increases the risk of decompression sickness, making it too dangerous for him to fulfil his passion.
James’ condition is called a patent foramen ovale (PFO) – an opening between the upper chambers of the heart. This normally closes soon after birth but in around one in four people it remains open.
Traditionally, patent foramen ovale are closed using a permanent metal device. But James carries a mutated gene which may put him at risk of developing a condition that affects the heart muscle, making it harder for the heart to pump blood (cardiomyopathy). This means he may require future treatments. A permanent metal implant could have made those procedures more difficult, or even impossible.

Cardiologists Carles Bautista, Alain Fraisse, Xiangbin Pan
Instead, the specialist heart teams at Royal Brompton Hospital and Evelina London Children’s Hospital, both part of Guy’s and St Thomas’, used a new biodegradable closure device called MemoSorb.
It seals the hole immediately, and then slowly dissolves over about a year, while the body’s own tissue grows naturally and permanently over the repair.

James has diving dreams
James said: “Before my operation I felt nervous, but mainly excited because it could mean I’m finally able to scuba dive. That’s my dream. I have a real passion for marine biology.
“I was also excited because no one else in the country has what I have in my heart. I feel really lucky I could have this device fitted to help me.”
The procedure lasted around 2 hours, led by consultant paediatric cardiologists Prof Alain Fraisse and Dr Carles Bautista, working closely with the international team who developed the device.
The flexible 28mm device was folded into a thin tube (catheter), inserted through the vein in the groin, and guided to the heart. Once in the correct position it was gently released to seal the hole.
James was back at school in Woking just one week after the procedure and has already returned to doing the things he loves, including art, Scouts and young archaeologists’ club.
While he can continue snorkelling, James will have testing in a few months to confirm the hole is completely sealed before he can dive. If all is well, he could finally receive the green light to start scuba diving.

James with dad Del and mum Suzanne
James’ mum Suzanne Hoddinott said: “We feel exceptionally fortunate to have had this opportunity. We can already see benefits since the procedure. James has grown, he’s more hungry and he’s got more energy. He’s definitely swimming faster.
“So far it’s looking really positive, and he’s already thinking ahead to when he can test for scuba diving.”
MemoSorb is made from biomedical polymers, including polydioxanone (often used in dissolving stitches) and a membrane of Poly-L-lactic acid.
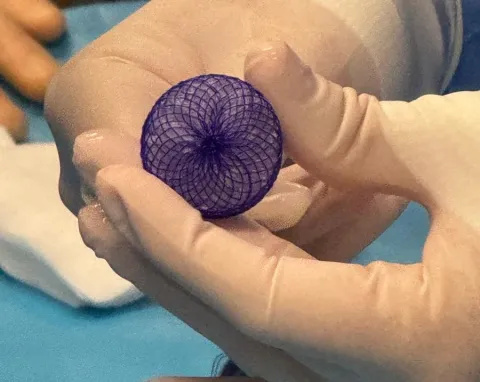
MemoSorb device
Prof Fraisse said: “We needed to close the hole in James’ heart, but a permanent metal implant wasn’t ideal — he may need future treatments involving the left side of his heart.
“MemoSorb has been used successfully in thousands of patients in China, and we believed this was the best option for James. He has made an excellent recovery and we’re hopeful for a positive long-term outcome.”
Although the device does not yet have FDA approval or a CE mark, the Medicines and Healthcare products Regulatory Agency (MHRA) authorised its use for James following approval by the Trust.
UK-based distribution company Revasc worked with Guy’s and St Thomas’ to enable James’ procedure.
Adam Lamb, National Sales Manager at Revasc, said: “At Revasc, bringing meaningful innovation to UK patients is something we take extremely seriously. This case perfectly reflects why we exist — to work in close partnership with clinicians to support solutions that can genuinely improve patient outcomes, particularly where existing technologies may limit future treatment options.
“We’re incredibly proud to have been trusted by Professor Fraisse and the team to support this procedure, and we look forward to helping make next-generation technologies available to more patients across the UK in the coming year.”
Last updated: February 2026
Contact us
If you're a journalist and have a media enquiry, please contact us.
Phone: 020 7188 5577
Email: [email protected]



